Equally individuals and businesses that get the job done with arXivLabs have embraced and approved our values of openness, Neighborhood, excellence, and person data privateness. arXiv is committed to these values and only works with companions that adhere to them.
Sterilization is a process for making a product sterile. Sterilization is completed by the subsequent strategy [2]:
Understand the assorted pyrogen testing techniques offered, the advantages & down sides of our Alternative, and decide if we can easily be an acceptable partner to suit your needs.
Test tube racks to hold the tubes and/or incubate dilution and reaction tubes. Micropipettes or disposable pipette ideas freed from interfering endotoxins and glucans are advisable. Vortex-style mixer, Para film (American Nationwide Can™) and warm-air oven While using the capacity to heat to not less than 250°C for depyrogenation of glassware.
This assistance won't include the entire matter of pyrogen and endotoxins testing. As an alternative, it addresses Individuals troubles Which may be topic to misinterpretation and so are not lined in compendial techniques or in currently available assistance documents. It is best to already have a radical idea of these paperwork when working with this steerage.
LAL test is employed for the quality control of pharmaceutical/professional medical parenteral preparations. The observation the haemolymph (blood)in the Horseshoe crab can sort clot during the existence of bacterial endotoxins gave increase to your Limulus amoebocyte lysate (LAL) test.
The bacterial endotoxin test (Wager) is often a critical part of good quality Regulate (QC) testing. Testing products and solutions for the presence of bacterial endotoxins is usually a fundamental safety necessity from the pharmaceutical and biomedical industries which is done on Uncooked As well as in-process supplies and for the ultimate launch of injectable or implantable products. These QC tests need to comply with regulatory demands enforced by world wide regulatory businesses.
If you're looking to investigate biofilms, glance no even further than Ingredient - our staff of experts may help you realize your aims.
The aluminum articles need to be stated as follows: “Incorporates no more than __ µg/L of aluminum”. This utmost quantity of aluminum may be stated as the best one among the following three amounts: The very best amount for the batches created in the previous get more info 3 yrs The very best level for that latest 5 batches The utmost amount with regard to historic stages, but only until completion of creation of the 1st five batches following the successful date of July 26, 2004 The deal insert for all LVIs, SVIs, and PBPs Utilized in the preparation or administration of TPN products will have to consist of a warning assertion. This warning has to be contained in the “Warnings” segment from the labeling and will have to condition the following: “WARNING: This product or service is made up of aluminum that may be toxic. Aluminum may perhaps arrive at toxic concentrations with prolonged parenteral administration if kidney purpose is impaired. Premature neonates are especially in danger since their kidneys are immature, they usually require significant quantities of calcium and phosphate options which comprise aluminum. Investigate implies that individuals with impaired kidney purpose, like premature neonates, who obtain parenteral levels of aluminum at increased than four to five µg for each kg a day accumulate aluminum at amounts associated with central nervous process and bone toxicity. Tissue loading may possibly manifest at even lower costs of administration of TPN goods and on the lock-flush alternatives utilized within their administration.”
The rFC assay includes a sensitivity of 0.005 EU/mL which is executed utilizing a artificial reagent which is made up of a recombinant form of Issue C that has been built in vitro. The assay will not be prone to Bogus positives as a result of beta-glucans, which originate from cellulose and other plant-primarily based products, because the BETs are.
These procedures contain the LAL gel clot technique, the turbidity assay process read more plus the kinetic chromogenic LAL test. The usage of human full blood and ELISA method may also be utilized for detecting the presence of pyrogens in the supplied products.
Sample template on how to create your investigation achievements and effects when implementing for a fellowship or grant
We design and style microbiology reports to incorporate the testing parameters and isolates required to achieve your required enhancement options. Wanting to begin? Click the orange "Get more information" button down below and complete the form. A member of our group will probably be in contact with you.
Exactly where the Assay within a monograph delivers a course of action to the Assay planning, where the overall withdrawable contents are to generally be withdrawn from only one-dose container with a hypodermic needle and syringe, the contents are to be withdrawn as completely as possible into a dry hypodermic syringe of a rated potential not exceeding thrice the amount to get withdrawn and fitted having a 21-gauge needle not less than two.
 Shaun Weiss Then & Now!
Shaun Weiss Then & Now! Michael J. Fox Then & Now!
Michael J. Fox Then & Now! Freddie Prinze Jr. Then & Now!
Freddie Prinze Jr. Then & Now!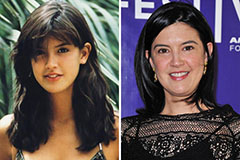 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Megyn Kelly Then & Now!
Megyn Kelly Then & Now!